Enrolment complete in Vogenx’ Phase 2 Trial of mizagliflozin for post-bariatric hypoglycaemia
- owenhaskins
- Feb 19, 2024
- 3 min read
Vogenx has completed patient enrolment of a mid-Phase 2 clinical dose ranging study (VGX-001-012) evaluating mizagliflozin in patients diagnosed with post-bariatric hypoglycaemia (PBH). Study VGX 001-012 is a multi-centre, randomised, single-blind, placebo-controlled, dose-ranging and regimen-finding trial in patients diagnosed with PBH. The study is designed to identify dose strengths and frequency of dosing to be administered in future clinical studies. Tolerability, safety and pharmacodynamic response to mizagliflozin is assessed in subjects during weekly dosing periods and during a mixed meal tolerance test (MMTT).
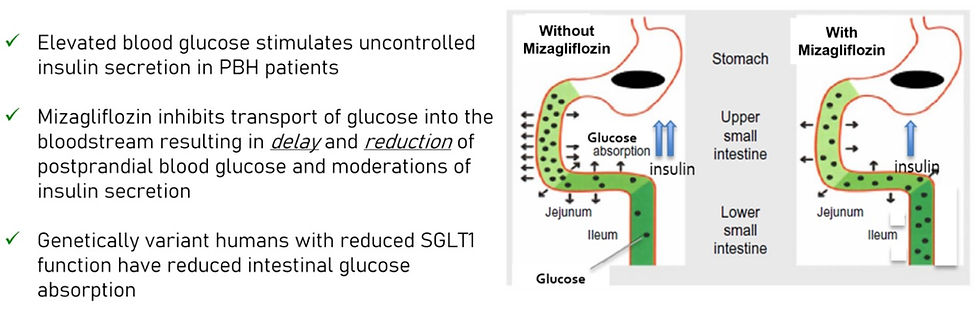
Mizagliflozin is a novel first in class small molecule therapeutic candidate being developed for PBH and gastroparesis. Mizagliflozin is an orally available small molecule inhibitor of the sodium-dependent glucose transporter-1 (SGLT-1), and has been administered to over 500 subjects in clinical studies and has shown statistically significant reductions in postprandial glucose absorption, insulin secretion and glucose-dependent insulinotropic polypeptide (GIP).
“Completing enrolment of this study is a significant step forward as we look to advance mizagliflozin as a first in class treatment to help patients better manage their symptoms and the daily challenges they present,” said Vogenx Chief Scientific Officer Bill Wilkison. “We look forward to seeing the data from study 012 and are thankful for the high level of engagement from our study investigators, their staff, and our patient participants who are all committed to making this study a success.”
Vogenx has previously announced positive results from study VGX 001-011, a phase 2 multi-centre, randomised, sequential crossover, single ascending dose study evaluating mizagliflozin in patients who suffer from PBH. The study examined four doses of mizagliflozin in patients randomly assigned to one of two treatment arms. Safety, tolerability and pharmacodynamic response to mizagliflozin were assessed during a MMTT.
Nine patients each received a baseline MMTT and were randomised to one of two treatment arms. Treatment Arm A received a 2.5 and 5.0mg mizagliflozin capsule at sequential visits. Treatment Arm B received a 2.5mg mizagliflozin liquid formulation and 10.0mg mizagliflozin capsule at sequential visits. Mizagliflozin doses were administered 20 minutes prior to MMTT administration. Pharmacodynamic samples were taken at various times from 0‑180 minutes, and glucose and insulin profiles determined.
The primary endpoints were safety and change in glucose nadir from baseline. Secondary endpoints included change from baseline peak plasma glucose and peak insulin. Levels of glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP) were examined as exploratory endpoints in a subset of patients.
In patients experiencing hypoglycaemia at baseline with a glucose nadir <70 mg/dL, the change from baseline mean glucose nadir for all capsule doses was 19.3 ± 14.7mg/dL (p=0.013); patients with glucose nadir at baseline ≥70mg/dL showed a change from baseline mean glucose nadir for all capsule doses of 6.0 ± 27.9mg/dL (p=0.59). These data suggest mizagliflozin is effective in preventing hypoglycaemic events without significantly impacting blood glucose levels in patients not experiencing hypoglycaemia.
In all subjects, the mean glucose nadir change from baseline for all capsule doses was 12.6±22.5mg/dL (p=0.045). The mean peak glucose change from baseline for all capsules was -29 ±36.4mg/dL (p=0.027). The mean peak insulin change from baseline for all capsules was -165 ±237uU/ml (p=0.012). In the subset analysis, mizagliflozin showed no significant effect on circulating GLP-1. In contrast, all mizagliflozin treatments in the subset analysis showed decreased peak GIP levels and AUC0-3h compared to baseline.
These data are consistent with previous clinical studies that showed mizagliflozin was safe and well tolerated. In addition, mizagliflozin is effective in preventing hypoglycaemia in PBH patients, by reducing both postprandial peak glucose and peak insulin. These results are strongly supportive of further development of mizagliflozin as an orally administered treatment for PBH.






Comments