Vogenx announces positive results from second phase 2 study of mizagliflozin in post-bariatric hypoglycaemia
- owenhaskins
- Jun 28, 2024
- 2 min read
Vogenx has announced positive results from study VGX 001-012 evaluating mizagliflozin in patients diagnosed with post-bariatric hypoglycaemia (PBH), suggesting mizagliflozin is effective in preventing hypoglycaemic events without significantly impacting blood glucose levels in patients not experiencing hypoglycaemia.

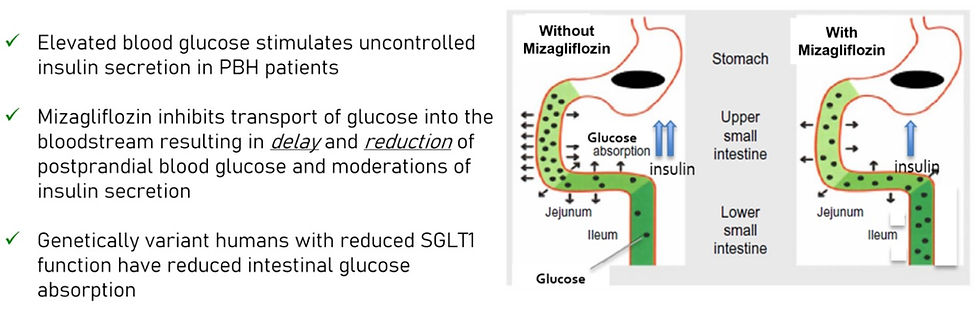
Mizagliflozin is an investigational first-in-class, oral, small molecule drug candidate that reduces postprandial glucose absorption and secretion of insulin and gastric inhibitory peptide, also known as glucose-dependent insulinotropic peptide (GIP). Through this mechanism, mizagliflozin has demonstrated statistically significant improvements in postprandial peak glucose and glucose nadir while reducing secretion of insulin and GIP, thereby reducing postprandial reactive hypoglycaemia in patients diagnosed with PBH.
The multi-centre, randomised, single-blind, placebo-controlled, dose-ranging and regimen-finding study enrolled 15 patients actively struggling with symptoms of PBH. Each patient was dosed with two doses of mizagliflozin and placebo, each for seven days (21-day study period) with a one-week washout period between regimens. Mixed meal tolerance tests were administered at the conclusion of each treatment period, and all patients wore blinded continuous glucose monitors throughout each treatment period.
The primary endpoints were safety and change in glucose nadir from placebo. Secondary endpoints included change from placebo peak plasma glucose and peak insulin.
In patients experiencing hypoglycaemia (<70 mg/dL) on placebo, the change from placebo mean glucose nadir for the 5.0 and 10.0mg doses combined (the highest doses examined) was 18.0 ± 7.75mg/dL (p=0.028; n=9); patients with placebo glucose nadir ≥70mg/dL showed a change from placebo mean glucose nadir for the 5.0 and 10.0mg doses of -7.97 ± 6.22mg/dL (p=0.24; n=9). These data suggest mizagliflozin is effective in preventing hypoglycaemic events without significantly impacting blood glucose levels in patients not experiencing hypoglycaemia.
The mean peak glucose changes from placebo for the 5.0 and 10.0mg doses was ‑57.6 ± ‑47.2mg/dL (p=0.004; n=9). The mean peak insulin change from placebo for the 5.0 and 10.0mg doses was -156.5 ± -89.5uU/ml (p=0.074; n=9). In the exploratory analysis, mizagliflozin showed significant reductions of Level 3 hypoglycaemia, ‑0.27 (p=0.023) and -0.29 (p=0.07) events/day versus placebo for the 5 and 10mg dose, respectively.
Combined 5 and 10mg showed a reduction of ‑0.26 (p=0.003) events/day versus placebo. Additionally, 5 and 10mg doses exhibited a 30.3% and 75.5% reduction in Level 2 hypoglycaemic events from placebo as measured by blinded CGM.
Mizagliflozin was well-tolerated, with no drug-related serious adverse events or participant withdrawals. The most common adverse event was diarrhea which was considered mild to moderate.
“We are very pleased by the results from Study 012,” said Dr William Wilkison, Chief Scientific Officer at Vogenx. “Mizagliflozin treatment consistently led to clinically meaningful improvements in both glucose and insulin while demonstrating significant effects on Level 3 and Level 2 hypoglycemic events. We look forward to continuing the development of mizagliflozin as a treatment for PBH.”
Overall, mizagliflozin has been administered to over 500 subjects in clinical studies and has shown statistically significant




Comments