top of page
Browse by category
Search


Pfizer to acquire Metsera and its next-generation obesity portfolio
Pfizer and Metsera have entered into a definitive agreement under which Pfizer will acquire Metsera, a clinical-stage biopharmaceutical...


EU approves Novo Nordisk’s oral semaglutide
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has approved an update to Novo Nordisk’s...


XNY Medical’s ENDO NOVA Powered Stapler 75mm Reload enhances performance, usability and patient outcomes
Bariatric News spoke with Dr Carlos Gomez (International Specialist Center International Hospital of Colombia, Bucaramanga, Colombia)...


FDA accepts Rhythm’s sNDA for setmelanotide in acquired hypothalamic obesity
The FDA has accepted for filing the Rhythm Pharmaceuticals’s supplemental New Drug Application (sNDA) for setmelanotide seeking approval...


Novo Nordisk lowers cost of Ozempic to $499 per month for self-paying patients
Novo Nordisk has launched a new offer that enables self-paying, eligible, type 2 diabetes patients access to authentic, FDA-approved...


FDA approves Wegovy for MASH with moderate to advanced liver fibrosis
The FDA has approved Wegovy (semaglutide) injection 2.4 mg to treat adults with MASH with moderate to advanced liver scarring (fibrosis),...


OIKOS launches cultured dairy drink to help build and retain muscle mass during weight loss
OIKOS has introduced OIKOS FUSION, which according to the company is the first of its kind, nutrient-dense cultured dairy drink developed...


Allurion to increase focus on low-dose GLP-1 combination therapy and muscle mass maintenance
Allurion Technologies has revealed a strategic direction with an increasing focus on low-dose GLP-1 combination therapy, muscle mass...


Allurion submits final PMA module for Allurion Balloon to the FDA
Allurion Technologies has submitted the fourth and final module of the Pre-Market Approval (PMA) application to the FDA and additional,...

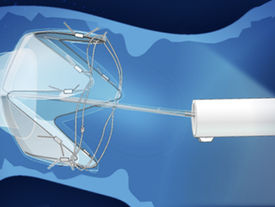
Morphic Medical gains CE Mark for RESET endoscopic device
Morphic Medical has received CE (Conformité Européenne) Mark approval for the RESET System, which according to the company is designed to...
Browse by tag






bottom of page

